MatrixCellect® 100 DBM Putty




MatrixCellect® 100 DBM Putty
MatrixCellect® 100 is a human bone derived, flowable demineralized bone matrix putty with no additional carriers, indicated for homologous use for the treatment of surgically created or traumatic skeletal defects.
- Overview
- Documents
- References
- Extremely irrigation resistant properties intended to aid in securing final allograft placement
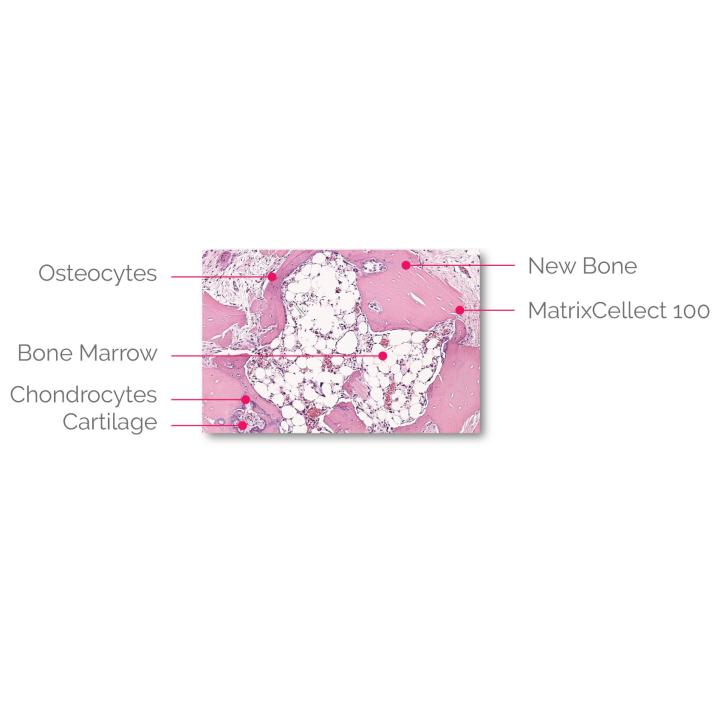
- In-vivo test results demonstrate all 5 bone forming elements present (chondrocytes, osteocytes, cartilage, bone marrow cells, and new bone)
- Every lot is verified for osteoinductivity post-sterilization
- Provided sterile via low dose, low temperature, gamma sterilization with a sterility assurance level (SAL) of 10-6
- Packaged with a shelf life of 2 years and may be stored in ambient temperatures
- Available in 1.0, 2.5, and 5.0cc syringes
900-00-081 Rev E
Trilliant is a distributor for CellRight Technologies.
® indicates U.S. trademark registration.
All trademarks and/or images are the property of their respective owners or holders.