EMPOWR Porous® Knee
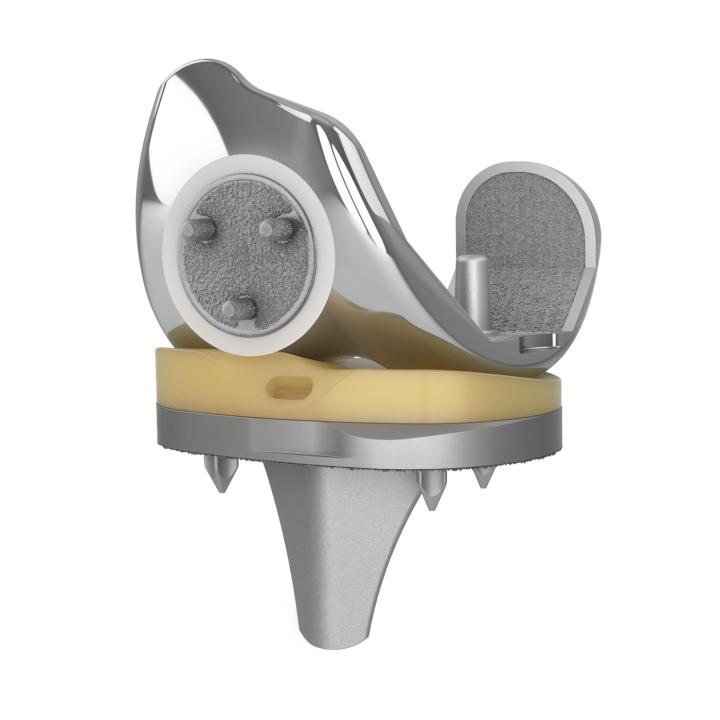








EMPOWR Porous™ Knee
The ONLY Dual-Pivot Knee Implant That Replicates Natural Joint Motion
The EMPOWR Porous Knee System is the culmination of decades of research and clinical experience resulting in data-driven materials optimized to enhance early fixation, while creating an ideal environment for immediate and long-term biologic fixation.1
- Overview
- References
FEMORAL COMPONENT
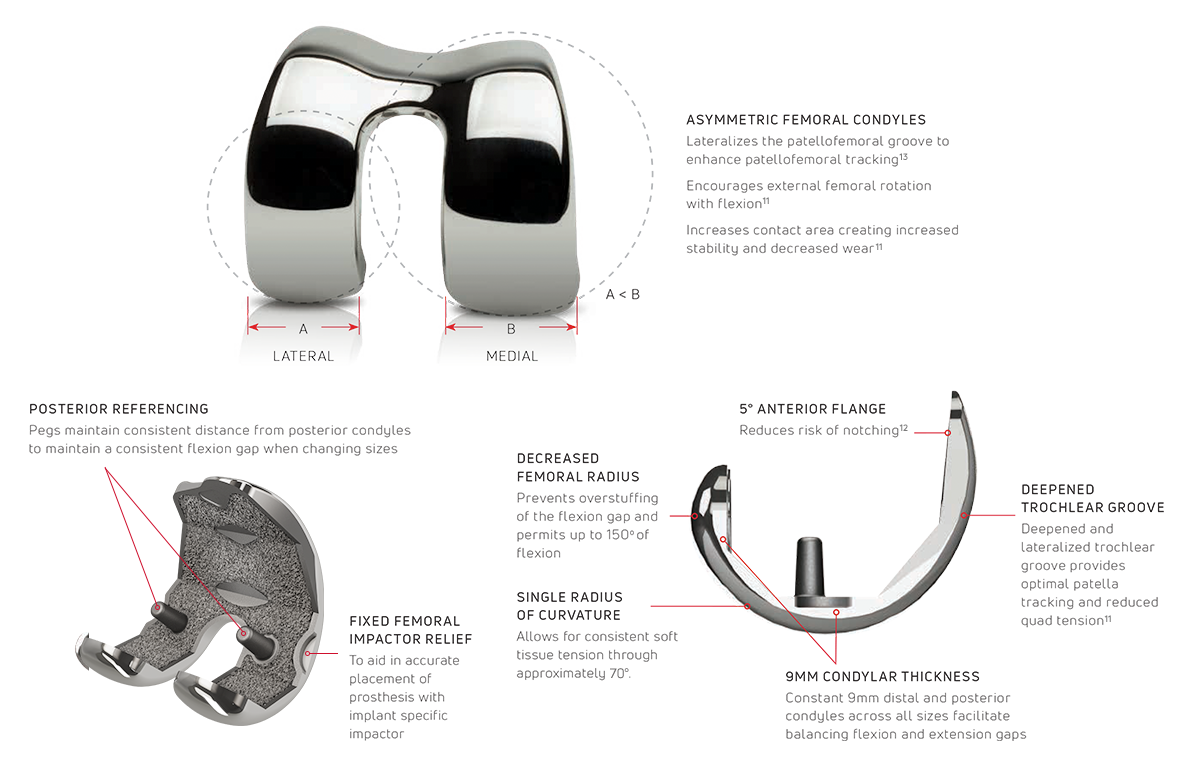
10 Asymmetric femoral components (20 total)
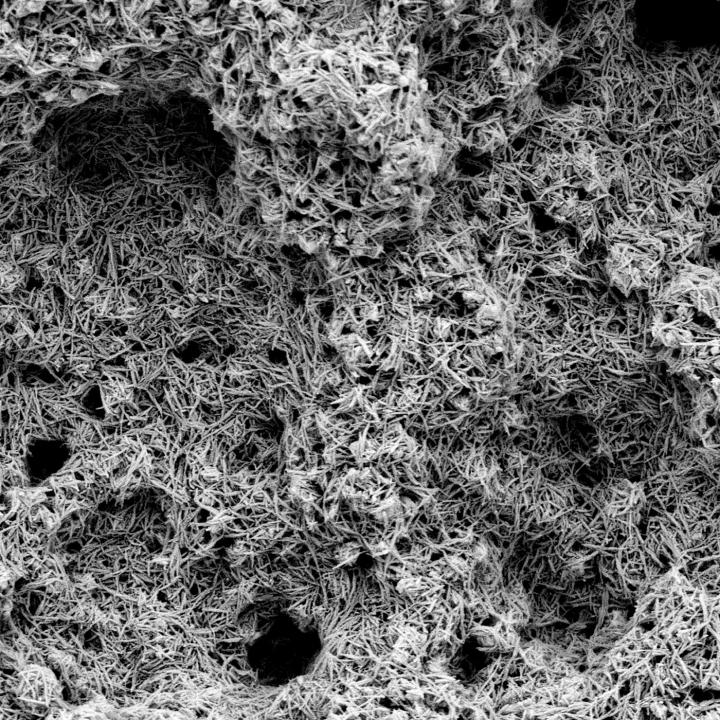
3D Matrix®
Non-spherical (roughened and irregular) sintered CoCr porous material with a strong clinical legacy.
EMPOWR Porous® Femur
- Shares all the of the features and benefits of the cemented EMPOWR 3D Femur while eliminating the need for bone cement.
- Supports long-term biological fixation
- Optimizes patellofemoral tracking and wear reduction
- Utilizes a CoCr porous coating (3D Matrix®)
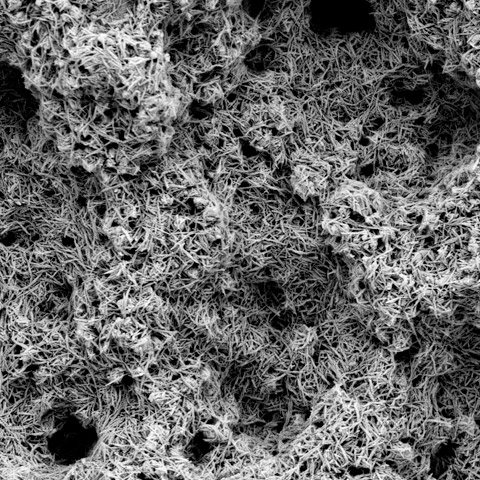
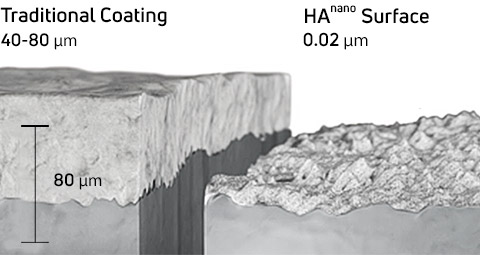
EMPOWR™ 3D Matrix® with HAnano® Surface
A 0.02 µm thin surface modification for implants composed of crystalline hydroxyapatite (HA) particles developed by Promimic.
Benefits
- Creates a bioactive surface for faster and stronger osseointegration.6
- Mimics the shape, composition, and structure of human bone tissue.7
- 1000x thinner than traditional implant coating.6
- Catalyzes biological response by improving the hydrophilicity of the implant which facilitates absorption of body fluids.7
- The results from more than 30 pre-clinical studies show that this surface accelerates osseointegration and enhances early bone growth.7
Applications
- Over 700,000+ clinical implantations in dental, spine, orthopedic, and cardiothoracic applications to date6, and is now available within the EMPOWR Porous® Knee System upon request.
- Can be applied to both traditional implant surfaces and 3D structures.®
- Can be used in combination with EMPOWR CoCr 3D Matrix®

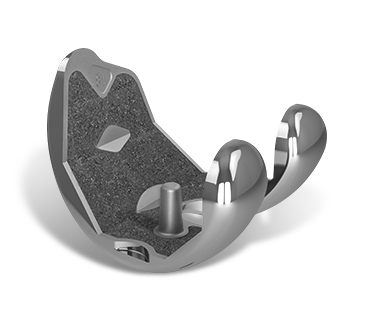
1. P2™ Porous Coating
Aids in the apposition of bone for superior in-growth results.1
2. Cruciform Pegs
Peripherally placed to provide initial component fixation and stability.2
3. Asymmetric Baseplate Design
Designed to maximize cortical coverage and prevent overhang.3
4. Tri-flange Bladed Keel
Bone sparing geometry optimized for cementless knee replacement. Designed to provide rotational stability and enhance initial fixation.3

Dual Pore Technology*
The industry’s only porous coating with two distinct pore sizes to optimize both immediate and long-term fixation.1
Superior Ratio Of Contact Area and Coefficient Of Friction
P2™’s large pores provide excellent initial fixation with an aggressive scratch fit. The small pores combined with increased contact area create an osteo-conductive environment which facilitates long-term biologic in-growth fixation.1
Scientifically Proven
P2™ is backed by the world’s largest metaphyseal animal study to ascertain Apposition Bone Index (ABI), Mineral Apposition Rate (MAR), % Bone In-growth, and Axial Pull-out Force.1


Porous Patella
Over-molded MXL e+ polyethylene articular surface with five sizes: 29, 32, 35, 38, and 41mm.
Similar to P2™ coating on the tibial baseplate, the Ti structure maximizes porous surface area and has the same mean porosity.10 The pegs & posterior surface have random cancellous-like porous Ti structure on them, designed for bony ingrowth.10
This Vitamin E cross-linked polyethylene reduces long-term wear, maximizes mechanical strength, and resists oxidation.8,9
*Large Pores - Pore size between each non-spherical bead (200-525 μm). Small Pores - Pore size within each non-spherical bead (25-65 μm).
- P2™ Testing Summary 0020327-001 Rev A 10/14
- 3D Knee™ Technical Monograph 0011102-004
- Bhimji, Safia, and R. Michael Meneghini. “Micromotion of cementless tibial baseplates: keels with adjuvant pegs offer more stability than pegs alone.” The Journal of Arthroplasty 29.7 (2014): 1503-1506.
- Meneghini, R. Michael, et al. “A dual-pivot pattern simulating native knee kinematics optimizes functional outcomes after total knee arthroplasty.” The Journal of Arthroplasty 32.10 (2017): 3009-3015.
- Mahoney, Ormonde M., et al. “The effect of total knee arthroplasty design on extensor mechanism function.” The Journal of Arthroplasty 17.4 (2002): 416-421.
- HAnano® surface research monograph on file with Promimic AB
- HAnano® surface brochure on file with Promimic AB
- DJO Surgical®. PR16-039-01 Empowr PS with Aged e+ UHMWPE Final Report. “Data on file at DJO Global. Laboratory testing does not necessarily indicate clinical performance.”
- Kurtz, Steven M. UHMWPE biomaterials handbook: ultra high molecular weight polyethylene in total joint replacement and medical devices. Academic Press, 2009
- Stereological Evaluation (ASTM F1854) – Pore size & porosity (Data on File)
- Melinda K. Harman, Stephanie J. Bonin, Chris J. Leslie, Scott A. Banks, and W. Andrew Hodge, “Total Knee Arthroplasty Designed to Accommodate the Presence or Absence of the Posterior Cruciate Ligament,” Advances in Orthopedics, vol. 2014, Article ID 178156, 8 pages, 2014.
- e+ testing data on file. Bench test results not necessarily indicative of clinical performance.
- “Replicating Native Knee Dual-Pivot Kinematics Improves Outcomes After Total Knee Arthroplasty.” R. Michael Meneghini MD - ICJR South Presentation June 2018