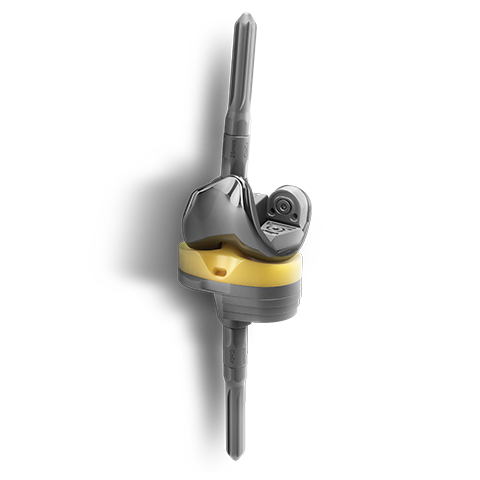
EMPOWR™
A culmination of research, clinical legacy and material technologies that have resulted in hip and knee products that help restore healthy kinematics and provide surgical efficiencies optimized for today’s health care environment.
NEW
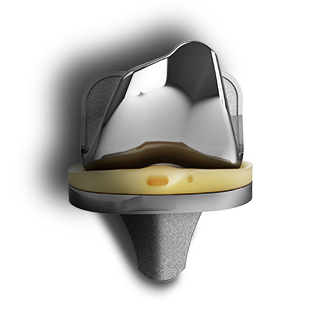
EMPOWR Revision Knee™
Revision knee surgery is demanding on you and your OR team - so we are redefining revision. EMPOWR Revision Knee offers streamlined instrumentation and stackable augments for both the tibia and femur which reduces inventory and OR clutter while the anatomic femoral boss reduces the need for offsetting and aids in flexion stability. With the addition of EMPOWR Revision Knee, we now offer a comprehensive system spanning from partial through revision knee arthroplasty.
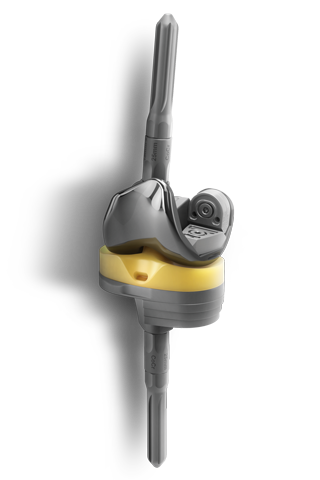
EMPOWR DUAL MOBILITY™
EMPOWR Dual Mobility™ is engineered for enhanced joint stability and helps reduce risks of dislocation by offering the largest assembled head for a given cup size1. Its robust locking mechanism aided by the dome peg and locking tabs helps build surgeon confidence by providing a visual and tactile liner engagement confirmation. These features, when combined with ONE tray instrumentation, provide a unique and efficient solution that a modern practice demands.
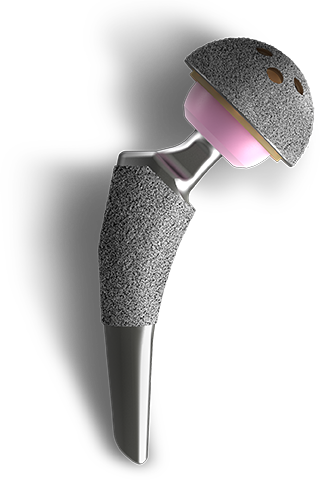
EMPOWR Acetabular® SYSTEM
EMPOWR Acetabular® is a modern, premium, intelligently designed acetabular system that features advanced fixation technologies and accepts both vitamin E polyethylene bearing and a dual mobility metal liner.
This system - combined with our femoral stems with a 12/14 robust micro-threaded trunnions – provides surgeons a comprehensive THA solution for all surgical approaches and settings.
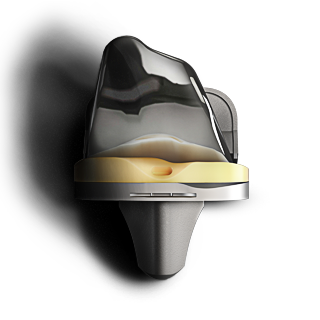
EMPOWR KNEE SYSTEM®
Based on more than a decade of extensive research – the EMPOWR 3D Knee® improves clinical outcomes.2,5 It is the first and only knee on the market that replicates natural kinematics with its dual pivot design.
Enovis’ proprietary P2™ coating on the EMPOWR Porous® Knee aids in the apposition of bone for excellent in-growth results.3
RESTORING
HEALTHY
Kinematics
Empowr Acetabular® System
Larger Head = More Stable Joint
14mm differential between femoral head and cup’s outer diameter, allowing for a larger head designed to aid in enhanced joint stability and greater range of motion.4
The addition of a dual mobility liner changes the differential to 10mm between the assembled femoral head and cup's outer diameter offering enhanced joint stability and helping reduce risks of intra-prosthetic dislocation1.
Empowr Knee System®
LESS PAIN WHEN WALKING
EMPOWR 3D Knee® patients had statistically significant less pain when walking compared to traditional non-conforming knees. These patients also participated in very active activities such as impact sports more regularly.5
Surgical Efficiencies
Empowr Acetabular® System
EMPOWR Partial Knee™
EMPOWR Dual Mobility™
LESS STORAGE COSTS.
LESS STERILIZATION COSTS.
ASC READY.
EMPOWR Acetabular® and Dual Mobility along with the EMPOWR Partial Knee have the ability to be implanted with just a single tray of instrumentation resulting in reduced space and sterilization costs savings aligning with the needs of the outpatient environment6.
Empowr Knee System®
Seamless
component
interchangeability
The EMPOWR Knee System® provides the surgeon with a comprehensive and versatile implant platform which allows for intra-operative flexibility when determining implant selection.
Advanced
Material
Technologies
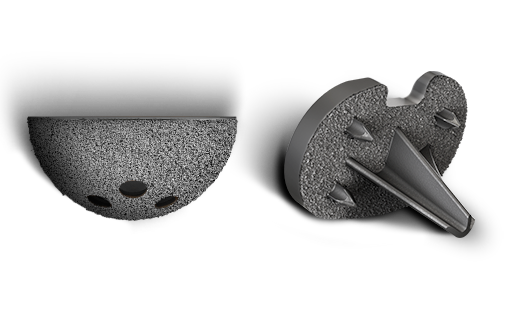
P2™
The industry's first and only Titanium Porous coating with two distinct pore sizes that aid in both immediate and long-term fixation.7
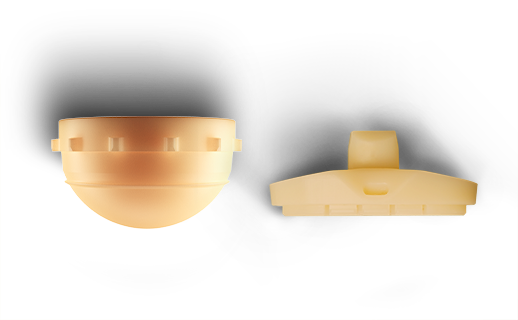
HXe+™ / e+™
A polyethylene material, e-plus, is blended with vitamin E and formulated to reduce long-term wear.8

3D Matrix®
Non-spherical (roughened and irregular) sintered CoCr porous material with a strong clinical legacy.

HAnano®
A 20 nanometer thin surface modification for implants composed of crystalline hydroxyapatite (HA) particles developed by Promimic that creates a unique bioactive surface for faster and stronger osseointegration.9
In the news
DJO® Introduces New EMPOWR Dual Mobility™ Hip System at AAOS
DJO, a subsidiary of Colfax Corporation (NYSE: CFX) and a leading global provider of medical technologies to get and keep people moving, today introduced its newest offering, EMPOWR Dual Mobility™, at the 2021 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS) (Booth #3435) Read More...
DJO® Introduces Two New EMPOWR™ Systems Designed To Accelerate Surgical Efficiency
The EMPOWR Acetabular™ System and EMPOWR Partial Knee™ restore healthy kinematics and promote surgical efficiency with single-tray implantation. Read More...
Clinical study shows DJO’s “dual-pivot” knee replacement technology reduces patient dissatisfaction, improves function and activity levels
Innovative data-driven design (3D) technology provides patients with a “more normal” feeling knee replacement. Read More...
DJO® unveils two additional products, further expanding the breadth of the EMPOWR Knee System®
EMPOWR™ Knee Platform now includes cementless primary and revision solutions for surgeons and patients Read More...
DJO® Announces Industry-First Risk Assessment Tool To Confirm Outpatient Joint Replacement Pathways
Risk stratification tool for Outpatient Total Joint Arthroplasty demonstrates improved predictive value for discharge to home, same day or next day Read More...
- Compared to Biomet G7, Stryker MDM, SNN OR30, whose head sizes are available in their respective Surgical Techniques on their corporate websites.
- “Replicating Native Knee Dual-Pivot Kinematics Improves Outcomes After Total Knee Arthroplasty,” R. Michael Meneghini MD - ICJR South Presentation June 2018.
- Data on file at DJO®. Laboratory testing does not necessarily indicate clinical performance.
- Burroughs, Brian R., et al. "Range of motion and stability in total hip arthroplasty with 28-, 32-, 38-, and 44-mm femoral head sizes: an in vitro study." The Journal of arthroplasty 20.1 (2005): 11-19.
- Sandberg, Rory, et al. "Dual-pivot bearings improve ambulation and promote increased activity levels in Total knee arthroplasty: A match-controlled retrospective study." The Knee 26.6 (2019): 1243-1249.
- Based on calculation of no. of trays compared to Oxford Uni Knee. https://www.zimmerbiomet.com/content/dam/zimmer-biomet/medical-professionals/000-surgical-techniques/knee/oxford-partial-knee-microplasty-instrumentation-surgical-technique.pdf
- P2™ Testing Summary 0020327-001 Rev A 10/14. Laboratory testing does not necessarily indicate clinical performance.
- e+™ Testing Data on file. Bench test results not necessarily indicative of clinical performance.
- HAnano® surface research monograph on file with Promimic AB