SpinaLogic Bone Growth Stimulation Research
Read Full Study:
Published in:
Medical & Biological Engineering & Computing 2015
Authors:
Michael R. Sheller, PhD
Sheller Biomedical Innovations Mesa, AZ
Timothy Stippick, BS
Escape Velocity Mechanical Design Phoenix, AZ
Key Points
- A computational anatomic model was developed for interbody and posterolateral spinal fusion procedures.
- EMS software was used to simulate the magnetic and electrical fields.
- From a biological standpoint, the fundamental property of magnetic fields allows CMF to stimulate all cells and their intracellular components within the treatment volume required for bone repair or consolidation.
- The therapeutic effect of the CMF SpinaLogic Bone Growth Stimulator is based on the action of combined magnetic fields, not an electric field.
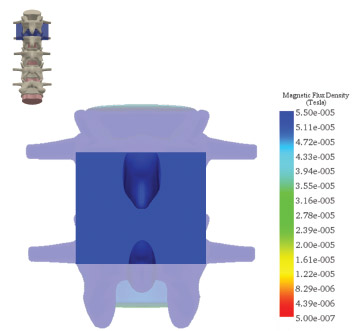
- The combined magnetic field generated by the CMF SpinaLogic device provides targeted and complete coverage of typical bone graft volumes for single and multi-level fusions of the L1-L5 vertebrae.
Combined Magnetic Fields Provides Full Coverage for Lumbar Spinal Fusion Procedures
Objective
Proprietary combined magnetic fields generated by the CMF SpinaLogic Bone Growth Stimulator were assessed to determine therapeutic coverage for bone graft volumes associated with typical lumbar spinal fusion procedure.
Methods
An anatomically based CAD model of the spine (L1-L5) was used to represent interbody and posterolateral fusion sites.
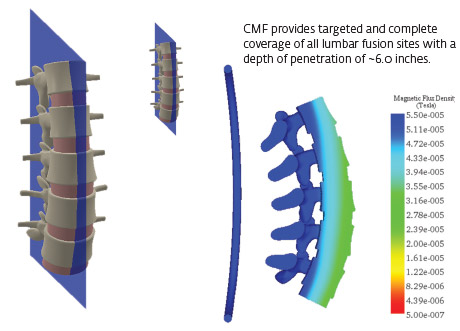
Computer simulations of the electromagnetic fields generated by the transducer coil were analyzed and measured to determine coverage of the modeled fusion sites.
Results
The field mapping data and simulation of the magnetic flux density (MFD) confirmed 100% coverage for typical posterolateral and interbody spine fusion sites.
Conclusions
The therapeutic effect of the CMF SpinaLogic bone growth stimulator can be attributed to the combined magnetic fields (not the electric field) generated by the device.
Consistent CMF Results for both Interbody and Posterlateral Procedures
CMF Provides 100% Coverage
Interbody Fusion Coverage
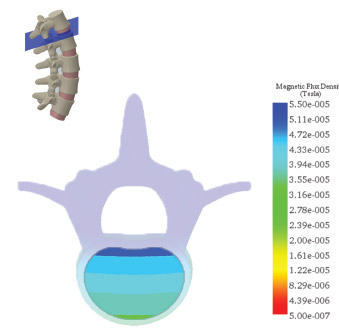
Posterolateral Fusion Coverage