DJO Global Announces Financial Results for Third quarter of 2014
SAN DIEGO, CA, November 11, 2014 – DJO Global, Inc. (“DJO” or the “Company”), a leading global provider of medical device solutions for musculoskeletal health, vascular health and pain management, today announced financial results for its public reporting subsidiary, DJO Finance LLC (“DJOFL”), for the third quarter ended September 27, 2014.
Third Quarter Results
DJOFL achieved net sales for the third quarter of 2014 of $305.5 million, reflecting growth of 6.1 percent, compared with net sales of $288.0 million for the third quarter of 2013. Net sales for the third quarter of 2014 were minimally impacted by changes in foreign currency exchange rates compared to the rates in effect in the third quarter of 2013.
Adjusted EBITDA for the third quarter of 2014 was $70.2 million, or 23.0 percent of net sales, reflecting 10.3 percent growth when compared with Adjusted EBITDA of $63.7 million, or 22.1 percent of net sales, for the third quarter of 2013.
The Company defines Adjusted EBITDA as net (loss) income attributable to DJOFL plus interest expense, net, income tax provision (benefit), and depreciation and amortization, further adjusted for certain non-cash items, non-recurring items and other adjustment items as permitted in calculating covenant compliance under the Company’s amended senior secured credit facilities (“Amended Senior Credit Facilities”) and the indentures governing its 8.75% second priority senior secured notes, its 9.875% and 7.75% senior notes and its 9.75% senior subordinated notes. Reconciliation between net loss and Adjusted EBITDA is included in the attached financial tables.
For the third quarter of 2014, DJOFL reported a net loss attributable to DJOFL of $21.2 million, compared to a net loss of $18.5 million for the third quarter of 2013. As detailed in the attached financial tables, the results for the current and prior year third quarter periods were impacted by significant non-cash items, non-recurring items and other adjustments.
"It is terrific to see our team deliver both strong accelerating revenue and Adjusted EBITDA growth in the third quarter. Building momentum in providing MotionCare™ products to keep arthritis sufferers moving, combined with strong global commercial execution, continues to drive increasing performance across most of our businesses,” said Mike Mogul, DJO's President and Chief Executive Officer.
“We are also pleased to report strong Adjusted EBITDA results for the third quarter with over 10% growth, due primarily to the contribution margin from increasing revenue and the impact of continuous improvement projects in both the cost of goods and operating expense areas,” said Mogul.
Sales by Business Segment
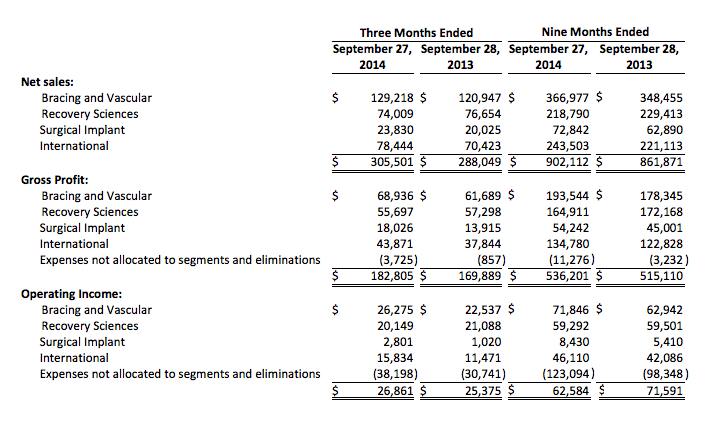
Net sales for DJO’s Bracing and Vascular segment were $129.2 million in the third quarter of 2014, reflecting growth of 6.9 percent, compared to the third quarter of 2013, driven by strong contribution from the sales of new products and improving sales execution.Net sales for DJO’s Recovery Sciences segment were $74.0 million in the third quarter of 2014, reflecting a contraction of 3.6 percent, compared to the third quarter of 2013, primarily due to the slower than anticipated sales in the EMPI and Regeneration business units.
Third quarter net sales within the International segment were $78.4 million. Excluding the impact of changes in foreign currency exchange rates from rates in effect in the prior year period, net sales for the third quarter of 2014 increased 11.3 percent from the third quarter of 2013.
Net sales for the Surgical Implant segment were $23.8 million for the third quarter of 2014, reflecting growth of 19.0 percent over net sales in the third quarter of 2013, driven by strong sales of each of the Company’s shoulder, knee and hip product lines.
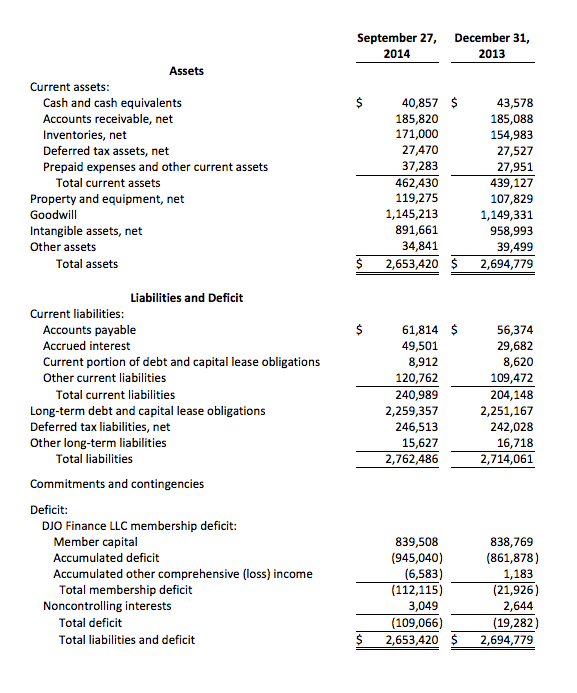
As of September 27, 2014, the Company had cash balances of $40.9 million and available liquidity of $89.5 million under its $100 million revolving line of credit.
Conference Call Information
DJO has scheduled a conference call to discuss this announcement beginning at 1:00 pm, Eastern Time today, November 11, 2014. Individuals interested in listening to the conference call may do so by dialing (866) 394-8509 (International callers please use (706) 643-6833), using the reservation code 22322226. A telephone replay will be available for 48 hours following the conclusion of the call by dialing (855) 859-2056 and using the above reservation code. The live conference call and replay will be available via the Internet at www.DJOglobal.com.
About DJO Global
DJO Global is a leading global developer, manufacturer and distributor of high-quality medical devices that provide solutions for musculoskeletal health, vascular health and pain management. The Company’s products address the continuum of patient care from injury prevention to rehabilitation after surgery, injury or from degenerative disease, enabling people to regain or maintain their natural motion. Its products are used by orthopedic specialists, spine surgeons, primary care physicians, pain management specialists, physical therapists, podiatrists, chiropractors, athletic trainers and other healthcare professionals. In addition, many of the Company’s medical devices and related accessories are used by athletes and patients for injury prevention and at-home physical therapy treatment. The Company’s product lines include rigid and soft orthopedic bracing, hot and cold therapy, bone growth stimulators, vascular therapy systems and compression garments, therapeutic shoes and inserts, electrical stimulators used for pain management and physical therapy products. The Company’s surgical division offers a comprehensive suite of reconstructive joint products for the hip, knee and shoulder. DJO Global’s products are marketed under a portfolio of brands including Aircast®, Chattanooga, CMF™, Compex®, DonJoy®, Empi®, ProCare®, DJO® Surgical, Dr. Comfort® and ExosTM. For additional information on the Company, please visit www.DJOglobal.com.
Safe Harbor Statement
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Such statements relate to, among other things, the Company’s expectations for its growth in revenue and Adjusted EBITDA and its opportunities to improve commercial execution and to develop new products and services. The words “believe,” “will,” “should,” “expect,” ”target,” “intend,” “estimate” and “anticipate,” variations of such words and similar expressions identify forward-looking statements, but their absence does not mean that a statement is not a forward-looking statement. These forward-looking statements are based on the Company’s current expectations and are subject to a number of risks, uncertainties and assumptions, many of which are beyond the Company’s ability to control or predict. The Company undertakes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. The important factors that could cause actual operating results to differ significantly from those expressed or implied by such forward-looking statements include, but are not limited to: the successful execution of the Company’s business strategies relative to its Bracing and Vascular, Recovery Sciences, International and Surgical Implant segments; the continued growth of the markets the Company addresses and any impact on these markets from changes in global economic conditions; the successful execution of the Company’s acquisition strategies; the impact of potential reductions in reimbursement levels and coverage by Medicare and other governmental and commercial payors; the Company’s highly leveraged financial position; the Company’s ability to successfully develop, license or acquire, and timely introduce and market new products or product enhancements; risks relating to the Company’s international operations; resources needed and risks involved in complying with government regulations; the availability and sufficiency of insurance coverage for pending and future product liability claims, including multiple lawsuits related to the Company’s cold therapy products and its discontinued pain pump business; and the effects of healthcare reform, Medicare competitive bidding, managed care and buying groups on the prices of the Company’s products. These and other risk factors related to DJO are detailed in the Company’s Annual Report on Form 10-K for the year ended December 31, 2013, filed with the Securities and Exchange Commission on February 25, 2014. Many of the factors that will determine the outcome of the subject matter of this press release are beyond the Company’s ability to control or predict.
DJO Finance LLC
Unaudited Condensed Consolidated Statements of Operations
(In thousands)
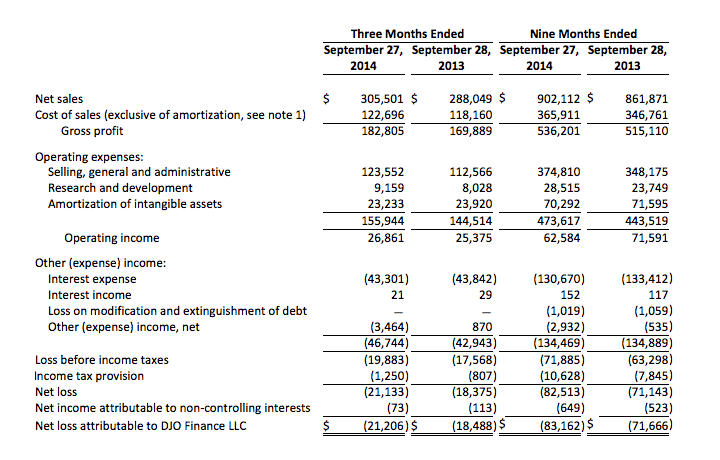
Note 1 — Cost of sales is exclusive of amortization of intangible assets of $8,645 and $25,980 for the three and nine months ended September 27, 2014 and $8,809 and $26,368 for the three and nine months ended September 28, 2013, respectively.
DJO Finance LLC
Unaudited Condensed Consolidated Balance Sheets
(In thousands)
DJO Finance LLC
Unaudited Segment Information
(In thousands)
DJO Finance LLC
Adjusted EBITDAFor the Three and Nine Months Ended September 27, 2014 and September 28, 2013
(unaudited)
Our Amended Senior Secured Credit Facilities, consisting of a $889.0 million term loan and a $100.0 million revolving credit Facilities, under which $10.0 million was outstanding as of September 27, 2014, and the Indentures governing our $330.0 million of 8.75% second priority senior secured notes, $440.0 million of 9.875% senior notes, $300.0 million of 7.75% senior notes, and $300.0 million of 9.75% senior subordinated notes represent significant components of our capital structure. Under our Amended Senior Secured Credit Facilities, we are required to maintain a specified first lien net leverage ratio, which is determined based on our Adjusted EBITDA. If we fail to comply with the first lien net leverage ratio under our Amended Senior Secured Credit Facilities, we would be in default. Upon the occurrence of an event of default under the Amended Senior Secured Credit Facilities, the lenders could elect to declare all amounts outstanding under the Amended Senior Secured Credit Facilities to be immediately due and payable and terminate all commitments to extend further credit. If we were unable to repay those amounts, the lenders under the Amended Senior Secured Credit Facilities could proceed against the collateral granted to them to secure that indebtedness. We have pledged a significant portion of our assets as collateral under the Amended Senior Secured Credit Facilities. Any acceleration under the Amended Senior Secured Credit Facilities would also result in a default under the Indentures governing the notes, which could lead to the note holders electing to declare the principal, premium, if any, and interest on the then outstanding notes immediately due and payable. In addition, under the Indentures governing the notes, our ability to engage in activities such as incurring additional indebtedness, making investments, refinancing subordinated indebtedness, paying dividends and entering into certain merger transactions is governed, in part, by our ability to satisfy tests based on Adjusted EBITDA. Our ability to meet the covenants specified above will depend on future events, many of which are beyond our control, and we cannot assure you that we will meet those covenants.
Adjusted EBITDA is defined as net income (loss) attributable to DJO Finance LLC plus interest expense, net, income tax provision (benefit), and depreciation and amortization, further adjusted for certain non-cash items, non-recurring items and other adjustment items as permitted in calculating covenant compliance and other ratios under our Amended Senior Secured Credit Facilities and the Indentures governing our 8.75% second priority senior secured notes, 9.875% senior notes, 7.75% senior notes and our 9.75% senior subordinated notes. We believe that the presentation of Adjusted EBITDA is appropriate to provide additional information to investors about the calculation of, and compliance with, certain financial covenants and other ratios in our Amended Senior Secured Credit Facilities and the Indentures. Adjusted EBITDA is a material component of these calculations.
Adjusted EBITDA should not be considered as an alternative to net income (loss) or other performance measures presented in accordance with accounting principles generally accepted in the United States of America (“GAAP”), or as an alternative to cash flow from operations as a measure of our liquidity. Adjusted EBITDA does not represent net income (loss) or cash flow from operations as those terms are defined by GAAP and does not necessarily indicate whether cash flows will be sufficient to fund cash needs. In particular, the definition of Adjusted EBITDA under our Amended Senior Secured Credit Facilities and the Indentures allows us to add back certain non-cash, extraordinary, unusual or non-recurring charges that are deducted in calculating net income (loss). However, these are expenses that may recur, vary greatly and are difficult to predict. While Adjusted EBITDA and similar measures are frequently used as measures of operations and the ability to meet debt service requirements, Adjusted EBITDA is not necessarily comparable to other similarly titled captions of other companies due to the potential inconsistencies in the method of calculation.
The following table provides reconciliation between net loss and Adjusted EBITDA:
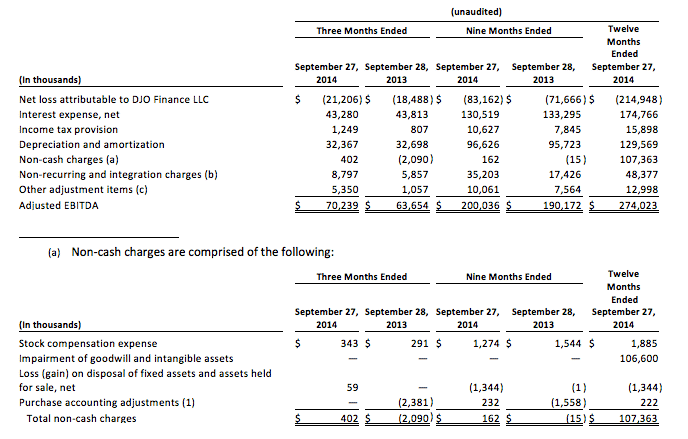
(1) Purchase accounting adjustments for the three months ended September 28, 2013 consist of $0.1 million of amortization of fair market value inventory adjustments, net of $2.5 million in adjustments to the contingent consideration for Exos.
(b) Non-recurring and integration charges are comprised of the following:
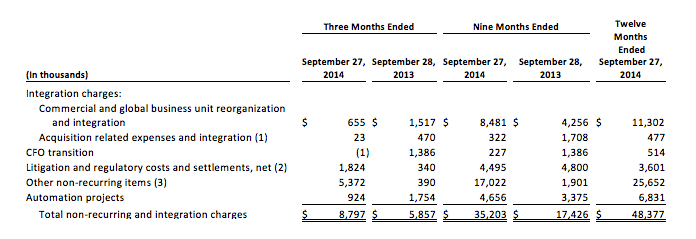
(1) Consists of direct acquisition costs and integration expenses related to acquired businesses and costs related to potential acquisitions.
(2) For the twelve months ended September 27, 2014, litigation and regulatory costs consisted of $0.7 million in litigation costs related to ongoing product liability issues related to our discontinued pain pump products, $4.8 million related to other litigation and regulatory costs and settlements, net of $1.0 million received related to an indemnity claim from a third party pain pump manufacturer and a settlement with its insurance carrier, and a $0.9 million favorable cost estimate adjustment for the post-market surveillance study required by the FDA related to our discontinued metal-on-metal hip implant products.
(3) For the twelve months ended September 27, 2014, other non-recurring items consist of $8.0 million in incremental Empi bad debt expense related to the Medicare CLBP decision, $12.6 million in specifically identified non-recurring operational and regulatory projects, $2.6 million in expenses related to our Tunisia factory fire and $2.4 million in professional fees and other non-recurring charges.
(c) Other adjustment items are comprised of the following:
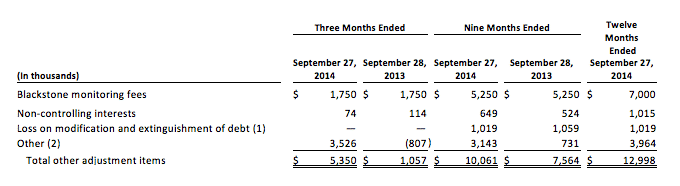
(1) Loss on modification and extinguishment of debt for the twelve months ending September 27, 2014 consists of $0.4 million of arrangement and amendment fees and other fees and expenses incurred in connection with the amendment of our senior secured credit facilities and $0.6 million related to the non-cash write off of unamortized debt issuance costs and original issue discount associated with the portion of our original term loans which were extinguished. Loss on modification and extinguishment of debt for the nine months ending September 28, ,2013 consists of $0.9 million in arrangement and amendment fees and other fees and expenses incurred in connection with the March 2013 amendment of our senior secured credit facilities and $0.2 million related to the non-cash write off of unamortized debt issuance costs and original issue discount associated with term loans which were extinguished.
(2) Other adjustments consist primarily of net realized and unrealized foreign currency transaction gains and losses.
